Effective method for removing nanoplastics and microplastics from water
Magnets could be used to remove plastic particles
Environmental pollution caused by microplastics and nanoplastics is a ubiquitous problem which cannot be tackled using currently available cleaning concepts. Researchers at FAU have now demonstrated how magnets could be used to remove plastic particles of various types and sizes from water.
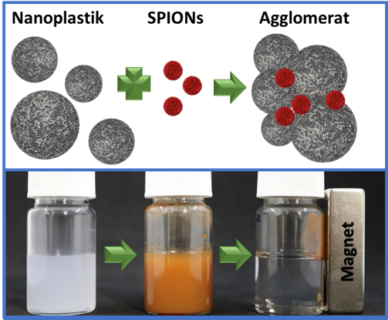
They used non-toxic, specially coated iron oxide nanoparticles known as SPIONs which they engineered specifically to bond with plastic surfaces. The SPIONs clump together with the plastic particles to form agglomerates which can then be easily collected using magnets thanks to the iron oxide they contain. The researchers have published their findings in the journal Materials Today.
Background knowledge: Microplastics and nanoplasticsMicroplastics have hit the headlines several times in recent years. They are known to be detrimental to our health, have been found everywhere from the Antarctic to the depths of the ocean, and although they have already been detected in organisms, are particularly prevalent in water. But what exactly are microplastics? What makes them so potentially dangerous, and how can we eradicate this man-made pollution. And what are nanoplastics?
|
Removing plastic from water using ‘smart rust’
In the current study, researchers from FAU have shown how plastic particles of various sizes and shapes can be removed from water using non-toxic, specially coated iron oxide nanoparticles. The FAU team of researchers led by Prof. Marcus Halik (Department of Materials Science and Engineering, Interdisciplinary Centre for Nanostructured Films (IZNF)), Prof. Dirk Zahn (Professorship of Theoretical Chemistry, Computer Chemistry Centre – CCC), Prof. Erdmann Spiecker (Department of Materials Science and Engineering, Centre for Nanoanalysis and Electron Microscopy (CENEM)) and Prof. Christoph Alexiou (Section for Experimental Oncology and Nanomedicine (SEON) at the Department of Otorhinolaryngology) used what are known as SPIONs (SuperParamagnetic Iron Oxid Nanoparticles).
Putting it simply, these materials could also be referred to as ‘smart rust’. The surface-modified SPIONs which are only approximately 30nm in diameter and therefore considerably smaller than the plastic particles which were investigated (100–970 nm) interact with the plastic particles, acting like glue, and stick them together to form larger aggregates. These aggregates of nanoplastics and iron oxide can then be easily removed from the water using magnets.
The groundbreaking approach behind this concept is the fact that due to surface functionalization the SPIONs can be adjusted to connect predominantly to specific types of plastics. The concept is flexible enough that it can be adapted to work efficiently with a wide range of nanoplastic mixtures, reflecting the situation in the environment.
Marco Sarcletti from Prof. Halik’s working group investigated the science behind these interactions, predominantly based on the opposite surface charges of the SPIONs and the plastic particles, and confirmed it using experiments. Simulations were used to back up the theory behind the concept (WG Zahn) and to investigate the morphology of the agglomerates in detail.
The experiments also clearly indicated that the special structure of the molecules on the SPION surfaces allow plastic particles to be differentiated from inorganic sediment particles. This is a critical point, ensuring that natural sediments do not reduce the efficiency of the cleaning process. In a test strategy developed specifically for magnetic nanoparticles, the researchers demonstrated that the SPIONs they use are not toxic (WG Alexious). This is also a fundamental aspect if the method is to be put to practical use in the future.
Naturally, this study was not able to answer all questions, and no technical procedure is suitable for cleaning micro and nanoplastics from the entire 1.4 billion km3 of water on Earth, but the team led by Prof. Halik is currently working on designing a technical procedure to scale up their method for cleaning water using magnets. Their aim is to minimise future contamination, which predominantly comes from rivers.
Their work has been funded by the German Research Foundation (DFG), the EAM Cluster of Excellence (Engineering of Advanced Materials) and the Graduate School Molecular Science (GSMS) at FAU as well as RTG 1896 and CRC 1411.
Further informationen
Prof. Marcus Halik
Tel.: 09131/85-70367
marcus.halik@fau.de
Marco Sarcletti
marco.sarcletti@fau.de