Bioactive glasses are able to heal infected bone defects and chronic skin wounds
Since January 2018, we have been presenting an exciting topic in the field of materials science every month.
The “topic of the month” is explained in a simple and understandable way and provides informative insights into the research activities of our department.
The topic of the month in February 2020 comes from the Institute for Biomaterials and has the title:
Bioactive glasses are able to heal infected bone defects and chronic skin wounds
by Dr. Kai Zheng
Worum geht es bei dem Thema?
Bioactive glasses (BGs), unlike those conventional glasses (e.g., window glass, glassware) used in daily life, are highly reactive and dissolvable in physiological fluids. They can induce specific therapeutic effects (e.g., promoted bone growth and blood vessel formation) through their dissolution products. Since the invention of the first BG (45S5 Bioglass) in the late 1960s, many BGs of novel compositions and morphologies (Figure 1) have been developed and widely applied in various biomedical applications, such as orthopedic/dental implant coatings, bone tissue scaffolds, and as building blocks of advanced medical devices for drug delivery, wound healing, and soft tissue repair.

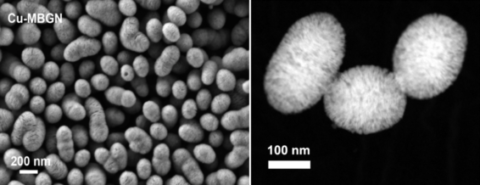
I have been involved in the EU-funded project “MOZART” as the representative of the Institute of Biomaterials (WW7, headed by Prof. Aldo R. Boccaccini). Recently I was awarded funding from the Emerging-Talents-Initiative (ETI) scheme funded by FAU. In these projects, we have developed novel mesoporous bioactive glass nanoparticles (MBGNs) that incorporate biologically active ions (boron, silver, copper, cerium) for treating delayed bone defects and chronic skin wounds. Because of their nanoscale particle size and large specific surface area (Figure 2), MBGNs have greater surface reactivity than conventional BGs. In addition, biomolecules (e.g., antibiotics, therapeutic proteins) can be effectively loaded into MBGNs due to the porous structure and large pore volume of these mesoporous nanoparticles. Recently, I am focusing on the control of the dissolution of MBGNs, in terms of modulating the dissolution rate and dissolution products, to achieve promoted biological responses, such as accelerated hemostasis and promoted angiogenesis.

Wo findet es Anwendung?
The developed MBGNs can be used as promising building blocks of medical devices for promoting the healing of delayed bone defects and chronic skin wounds. Due to bacterial infection or inflammation, the healing of some bone defects or wounds (e.g., those under diabetic conditions) can be delayed or even prevented. Conventionally treatments involving using antibiotics or anti-inflammatory drugs to help the healing of these defects or wounds, but these drugs can be denatured during the storage and administration. In addition, these drugs usually suffer from a burst release and cannot exert a long-term therapeutic effect. On the other hand, MBGNs are relevant stable in the physiological environment and could release therapeutic ions in a sustained and controlled way. Moreover, specific drugs can also be loaded into MBGNs and locally released to exert synergistic effects with ions towards promoted therapeutic effects. Therefore, MBGNs represent a type of stable, effective and inexpensive inorganic therapeutic agent. The developed MBGNs have been incorporated into different “smart” hydrogels (e.g., thermosensitive) to develop injectable composite hydrogels for treating infected bone defects or for healing inflammatory chronic skin wounds.
Was ist weiter geplant?
The developed MBGNs have shown promising in vitro cellular response. In vivo studies in rat defect models have also been performed in collaboration with Charité – Universitätsmedizin Berlin and the data are being analyzed. Considering the important roles of ionic dissolution products in bone regeneration and wound healing, MBGNs with controlled dissolution by carefully designing chemical composition are being investigated to achieve optimized therapeutic effects for tissue regeneration applications.
Zur Person:

I moved to Erlangen for pursuing my Ph.D. in 2012 after obtaining my Master’s degree at East China University of Science and Technology (Shanghai, China). In 2017 I successfully earned my Ph.D. (Dr.-Ing) under the supervision of Prof. Aldo R. Boccaccini at the Institute of Biomaterials (WW7), University of Erlangen-Nuremberg (FAU). Currently, I am a postdoctoral researcher at WW7. Outside the university, I like playing football, hiking and travel. I also enjoy reading history books, cooking, and playing video games.